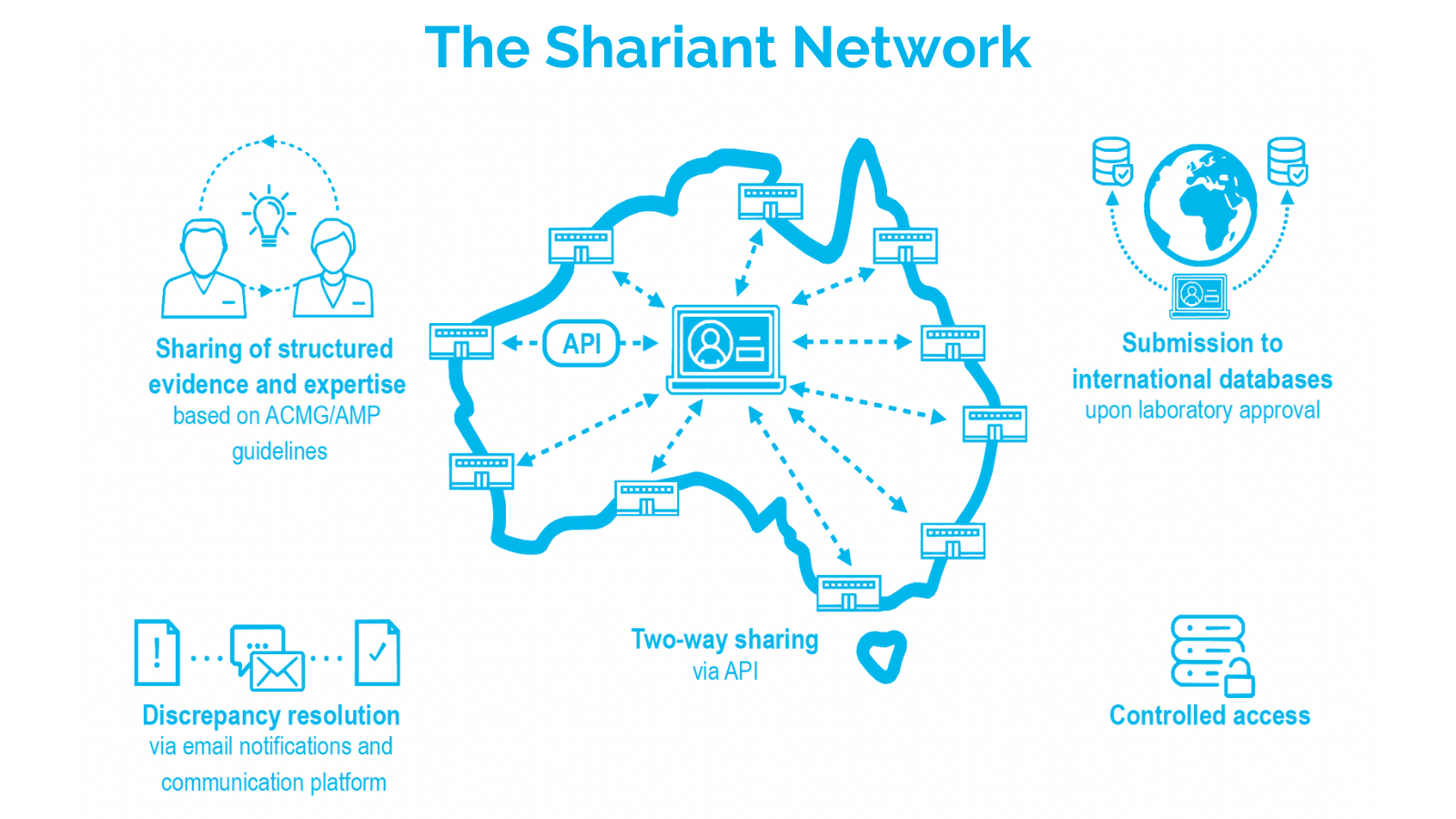
A national platform for diagnostic laboratories to share genetic evidence and build consistent interpretations of that information, is paving the way for more accurate diagnosis of patient conditions.
The variant hub and communication platform, Shariant, has been taken up by 11 laboratories in Australia, with more set to come on board in the next six months.
The project is an Australian Genomics initiative in collaboration with the Brisbane-based QIMR Berghofer Medical Research Institute and the Centre for Cancer Biology, an SA Pathology and University of South Australia Alliance. A tool developed by the Centre for Cancer Biology, VariantGrid, was the basis for developing the Shariant platform.
The Shariant story is described in the latest edition of the American Journal of Human Genetics where it reveals the complexities of interpreting the clinical data and the need for consistency.
Laboratories use information from external and internal sources to interpret if a variant is disease-causing, benign, or of uncertain clinical significance. Therefore, interpretation of variants can sometimes differ between laboratories, potentially leading to differences in clinical management advice between patients with the same variant.
One of the key outcomes of the project was establishing a process for laboratories to share knowledge about variant interpretations, and to promote consistent interpretation for newly discovered variants before a laboratory report is prepared.
“This is the first time a diagnostic laboratory in Australia has been able to share a classification with other laboratories, and reclassify a variant based on shared evidence,” said Project Lead Professor Amanda Spurdle of QIMR Berghofer.
“By gradually standardising interpretations nationally we can provide much more accurate diagnoses for patients irrespective of their location. Shariant now contains nearly 20,000 variant interpretations, and we expect this number to increase markedly.”
Shariant has also allowed Australian labs to share variant interpretations internationally, with a seven-fold increase in the number of variants submitted to the public database ClinVar from 2017 to August 2022 – an increase largely enabled by the platform.
Clinical Geneticist Professor Zornitza Stark said sharing information between laboratories was key to improving test results: “It means that doctors can provide faster and more accurate diagnosis to patients.”
“Shariant has massively scaled up data sharing between Australian laboratories and made it easy for us to contribute internationally,” she said.

