MitoMDT (Mito Multi-Disciplinary Team) will be a national Mitochondrial Diagnostic Network for Genomics and Omics, comprising clinicians, researchers and diagnostic scientists. We seek to combine genomic testing with additional Omic technologies to improve diagnostic rates for Mitochondrial Disease (MD) to over 70%. This will identify novel genes, mechanisms and phenotypes, while enabling personalised treatments and achieving better health outcomes for patients with Mitochondrial Disease.
Principal Investigator

Professor David Thorburn
Murdoch Children’s Research Institute
Recruitment information
Recruitment period: until May 2025
Inclusion criteria
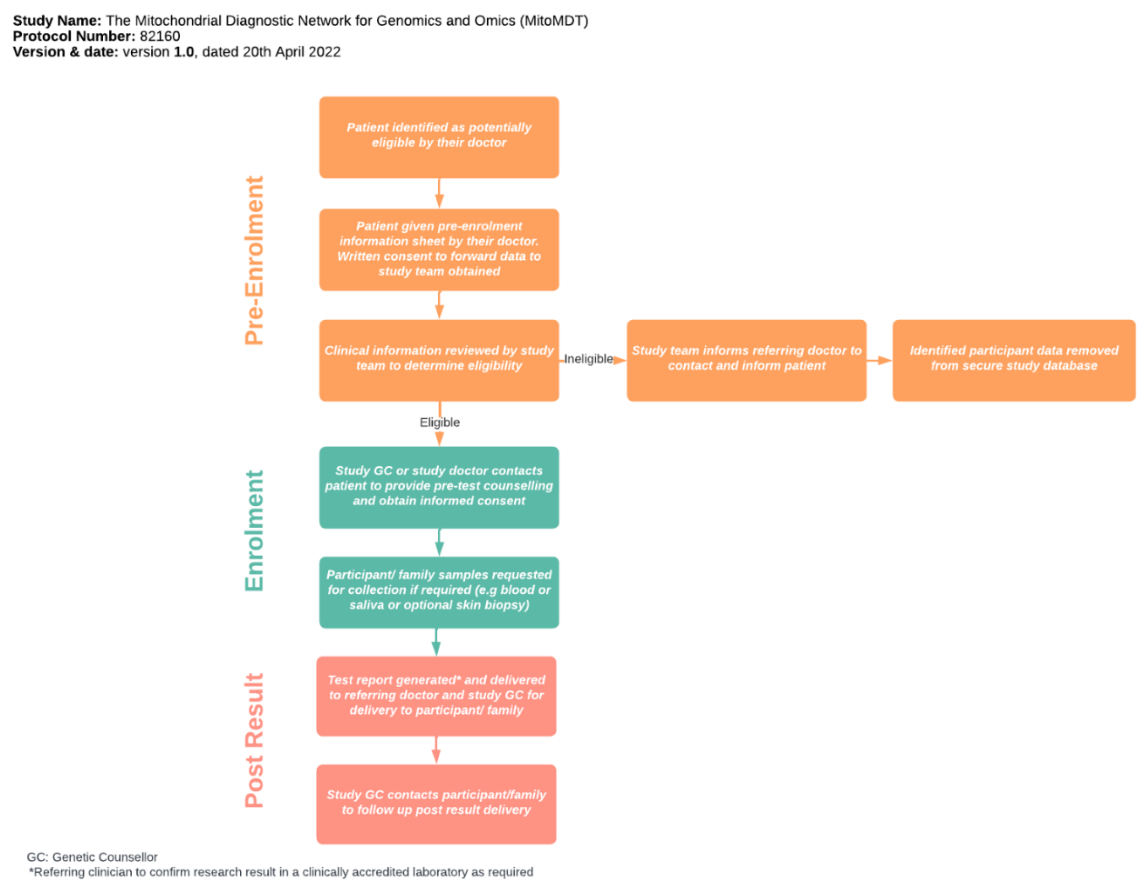
The MitoMDT study will assess participant eligibility based on clinical presentation, using modified Nijmegen Mitochondrial Disease Criteria (mNMDC). Referring clinicians are required to provide patient clinical information and demographic information via the study’s REDCap survey.
It is recommended that the clinicians discuss potentially eligible patients with the clinical lead in your state prior to submitting patients for consideration to the MitoMDT study.
Eligibility scores will be calculated based on the sum of:
- Clinical signs and symptoms (max. 4 points), comprising Muscle presentation (max. 2 points), CNS presentation (max. 2 points), Multisystem disease (max. 3 points)
- Metabolic / Imaging studies (max. 4 points)
- Tissue pathology / Biochemistry / Genetics (max. 4 points)
Patients will usually only be eligible with a total mNMDC score of 5 or more, with 5 to 7 classed as a probable mitochondrial disorder and 8 to 12 classed as a definite mitochondrial disorder. Patients with a score <5 can be considered if the MitoMDT review agrees that a strong suspicion of mitochondrial disease can be justified. Note that each mNMDC category (and clinical sub-categories) has a maximum score.
Exclusion criteria
- Participants with a non-mitochondrial disease diagnosis explaining phenotype from other investigatory testing
- Participants with a confirmed molecular diagnosis
Sample requirements
| Required Samples | Optional Samples |
| A blood sample
A saliva sample (if blood cannot be obtained) A urine sample |
Skin biopsy |
| Muscle biopsy
Liver biopsy (if other surgery is planned) |
Referral process & recruitment sites
Recruitment sites
| State/Territory | Clinical Site |
| VIC
|
Royal Children’s Hospital Melbourne/ Murdoch Children’s Research Institute; MCRI
(Victorian Clinical Genetics Services and Metabolic Department) |
| NSW
|
Children’s Hospital at Westmead and Sydney Children’s Hospital Randwick Clinical Genetics Services
Children’s Hospital Westmead Metabolic genetics service |
| Northern Sydney Local Health District
Royal North Shore Hospital
NeuRa – Randwick Neuroscience Research Department |
|
| QLD
|
Children’s Health Queensland Hospital and Health Service
Queensland Children’s Hospital
|
| WA
|
Royal Perth Hospital
|
Child and Adolescent Health Service Perth Children’s Hospital
|
Contact
Amanda Samarasinghe
Amanda.samarasinghe@mcri.edu.au
03 9345 9473