Australian Genomics has updated the previously released clinical consent package (2019) to provide practice-informed clinical consent forms for genomic and genetic testing. These materials, released in 2024, meet current national standards and clinical practices. This revised package includes a health professional guide and patient fact sheet.
Over the next year, Australian Genomics will progress efforts to co-develop resources to support Aboriginal and Torres Strait Islander people through the consent process, along with customised consent forms for somatic testing for non-inherited genetic conditions and prenatal genomic testing.
Download our latest clinical consent form and supplementary information below.
Please ensure you have appropriate approval(s) if you decide to use these forms in your clinical practice.
Tissue-targeted (somatic) testing consultation
There is no standardised process for obtaining consent in tissue-targeted genomic testing at present, which can lead to inconsistencies in the patient experience and make it complicated for clinicians to share data and expand their knowledge about health conditions. Australian Genomics is seeking insights from stakeholders on newly developed clinical tissue-targeted (somatic) genomic consent package. The consultation is now open and relevant documents can be found below.
Participate in the consultation here.
Resources
Tools for patients
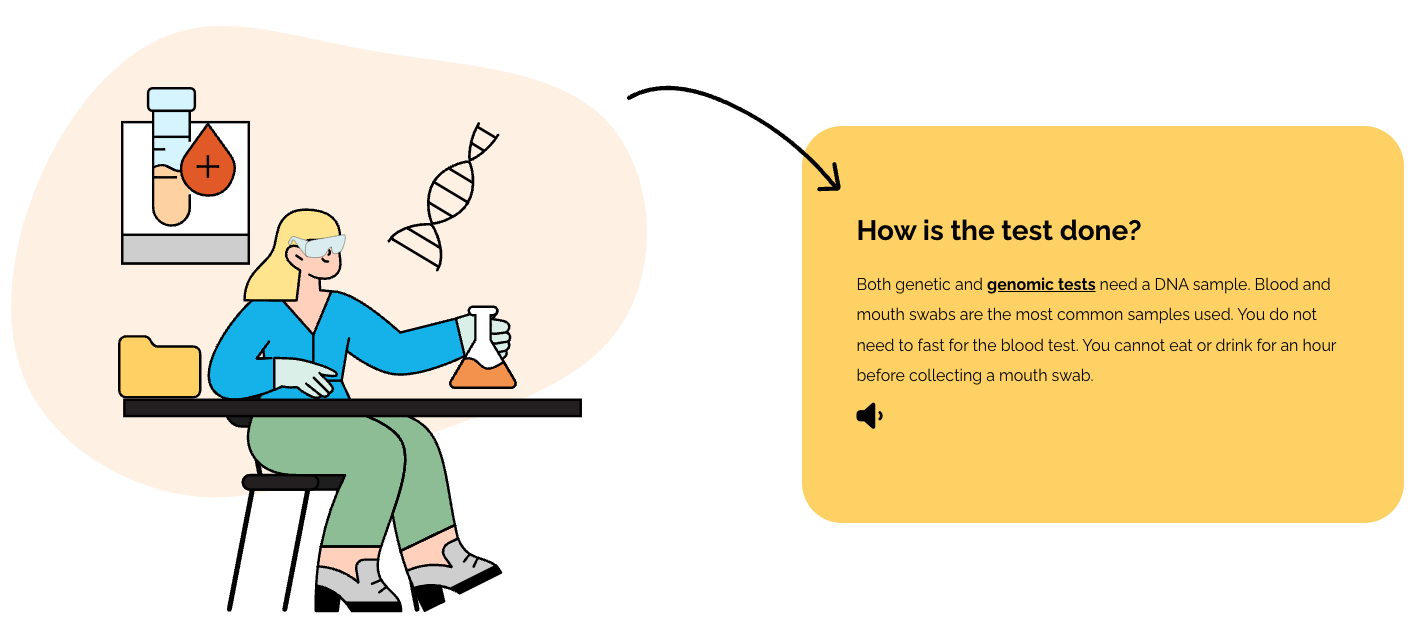
Genomic testing can be complicated. This interactive online tool provides easy and accessible information in bite-sized chunks to guide patients through the key concepts of genomic testing. This tool is designed to complement the updated clinical consent package above.
Contact
Keri Finlay

